行业资讯
ARTICLE FOR TRANSLATION INTO CHINESE
Originally published in English in:
原文以英语发表于:
Magazine: Manufacturing Chemist
杂志名:《制造化学师》
Issue: September 2014
发表日期:2014年9月
Title: Analytical strategies for the de-formulation of dry powder inhalers
标题:解析:如何有效解析干粉吸入剂配方
Author: Dr Paul Kippax, Pharmaceutical Portfolio Manager,
Malvern Instruments
作者:马尔文仪器公司制药产品经理PaulKippax博士
Our ref: MAL/JOB/2876a
本公司文号:MAL/JOB/2876a
Words: 1709
字数:1709
Figures x3 (Thumbnails only at end of document – high res files supplied separately)
图片:共3张(仅在文件末尾提供缩略图,高分辨率图片文件另行提供)
Figure 1: Powder concentration (Cv) versus Time profiles recorded for the emptying of a dry powder inhaler using laser diffraction. The data show that a higher concentration is delivered at high flow rates. This relates to the energy available for aerosolisation of this cohesive formulation.
图1:在干粉吸入器排空过程中,通过激光衍射技术获得的粉末浓度(Cv)与时间变化的关系。数据表明,当气体流速较高时,浓度也更高,而这与该粘性配方雾化时可提供的能量密切相关。
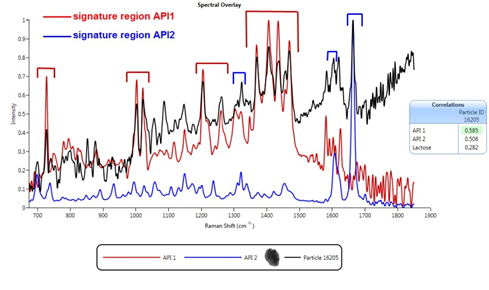
Figure 2: Comparing the Raman spectrum of individual particles with reference spectra for the APIs in the formulation enables secure chemical classification of the sample.
图2:将单独颗粒的拉曼光谱与配方中的API参考光谱进行比较,可对样本成分进行可靠的化学分类。
Figure 3: MDRS enables the precise classification of particles in an NGI sized fraction as being API, lactose or multicomponent agglomerates
图3:在使用新一代撞击器对样本进行粒径分级时,采用拉曼结合自动成像技术(MDRS)可将每一级别的颗粒准确分类为API、乳糖或含有多种成分的团聚物。
Analytical strategies for the de-formulation of dry powder inhalers
解析:如何有效解析干粉吸入剂配方
By DrPaul Kippax, Pharmaceutical Portfolio Manager, Malvern Instruments
作者:马尔文仪器制药产品经理Paul Kippax博士
The complexity of dry powder inhalers (DPIs) makes them one of the toughest generic development targets. In this article Dr Paul Kippax, Pharmaceutical Portfolio Manager for Malvern Instruments,reviews the guidance available from the FDA for DPI formulation development and discusses analytical strategies that can be employed for the process ofde-formulation of a complex DPI formulation.
干粉吸入剂(DPIs)的结构较为复杂,这使得它成为当下最难进行仿制研发的药品之一。而本文不仅回顾了美国食品药品监督管理局(FDA)对干粉吸入剂配方开发的指导原则草案,更探讨了多种可用于解析复杂干粉吸入剂配方的分析策略。
The performance of aDPIis affected bycomplex and subtle interactions between the drug substance, excipients and the device used.Arguably this makes DPIs a more challenging generic target than any other pharmaceutical product, a conclusion evident from the current commercial landscape.Advair, for example, one of the most successful DPIs to come to market, is now off-patent but relatively free of generic competition,despite well-funded efforts by a number of generic companies to access market share [1].
干粉吸入剂中的药物成分、赋形剂及所用设备之间常发生复杂而微妙的相互作用。这样的相互作用一方面能影响干粉吸入剂的性能表现;另一方面,也使得干粉吸入剂比其他药品更难进行仿制--目前商业药品市场的现状也恰好证明了这一点。例如,市场上最成功的干粉吸入剂产品之一--舒利迭(Advair)目前已过了专利保护期,但尽管许多仿制药公司都投入了大量的资金和精力,希望在该市场中分一杯羹,能与之匹敌的实在是少之又少。[1]
The FDA’s guidance for industry for the development of DPIs [2]emphasisesthe importance ofparticlecharacterisation. Successful drug delivery with a DPI relies on dispersing particles to a suitable size for deposition in the lung. Particle characterisation helps to elucidate and control the dispersion mechanisms associated with drug delivery, and supports the development of a product with robust aerosolisation behavior, controlled bioavailability and consistent clinical efficacy. Relevant and efficient particle characterisation strategies aretherefore critical in the development of both new and generic products.
FDA对干粉吸入剂开发的行业指导中[2]特别强调了颗粒表征的重要性。我们知道,若想成功传输干粉吸入剂,需要将颗粒分散成合适的大小,使其能在肺中沉积。而颗粒表征有助于分析并控制与药物传输相关的分散机制,确保开发出的产品具有高效的雾化作用和可控的生物利用度,并保证临床效果的一致性。因此,在新药和仿制药的开发过程中,相关度高且有效的颗粒表征方法至关重要。
This article assessesthe potential of a number of analytical techniques to accelerate thede-formulation of DPIs, focusingon the use of Morphologically Directed Raman Spectroscopy (MDRS)in conjunction with cascade impaction to probe the nature of large particles and agglomerates.
本文评估了几种能够加快干粉吸入剂配方解析的分析技术,并重点探讨如何用级联撞击器与拉曼结合自动成像技术(MDRS)相结合的手段来探测大颗粒和聚结物的性质。
Understanding DPIs
干粉吸入剂详解
The majority ofDPIsare classified as passive, with delivery of the dose beingdriven solely by the inhalation manoeuvre of the patient. As the patientinhales, air is drawn through the device, aerosolizing the powder doseand dispersing it to form drug particles of a respirable size. The size of the particles delivered to the patient, and the total emitted dose, depend on theenergy supplied by inhalation being sufficient todisperse the formulation with the chosen device.The FDA draft guidance emphasises how all aspects of the DPI -device configuration, packaging (which can be blister, capsule or bulk reservoir) and the physical properties of the formulation - impact this dispersion process.
由于干粉吸入剂只能依靠患者吸入才能完成输送,所以大部分的干粉吸入剂都被归类为被动式药物。患者吸气时,空气会通过吸气装置,使药物粉末雾化并分散成符合吸入尺寸的药物颗粒。而患者使用某一特定装置吸气时产生的能量将影响制剂的分散程度,进而决定药物粒径以及总的喷出剂量。而FDA起草的指南中详细列举了干粉吸入剂的设备结构、包装(如泡壳包装、胶囊或散装容器)以及配方的物理特性对分散过程的影响。
The size of the active pharmaceutical ingredient (API) particles received by the patientdirectly correlates with bioavailability in the lung. This is why delivered particle size, along with dose uniformity, is a critical parameter for DPI performance.A size range of 1-5 µm has been established as optimal for pulmonary delivery butparticles in this range tend to be highly cohesive.One of the central challenges of DPI development is to engineer a device/formulation combination that ensures successful dispersion of a cohesive dose using only the energy supplied by the inhaling patient.
患者摄入的活性药物成分(API),其颗粒大小直接关系到其在肺部的吸收程度。因此,干粉吸入剂的性能表现需要通过药物颗粒大小以及剂量均匀性来衡量。当前肺部给药的最优粒径范围为1- 5微米,但该范围内的颗粒往往具有较高的粘性。对干粉吸入剂进行开发时,研究人员面临的一个核心挑战就是要设计出一种设备/配方组合,以确保患者在吸气时就能均匀分散粘性药剂。
In many DPIs,efficient drug delivery is achieved by blending the API with a carrier particle. An alternative strategy is to engineer the particle properties of the API, such as shape, surface charge and roughness, to enable carrier-free delivery. De-formulation of a reference labeled drug (RLD)may therefore call for detailed analysis of a multi-component blend, and/or close scrutiny of the characteristics of the API.
对许多干粉吸入剂而言,有效的药物传输是通过混合API与载体颗粒实现的。但现在,我们还可以通过设计API的形状、表面电荷和粗糙度等颗粒性质,实现无载体传输。因此,对参考目录药物(RLD)进行配方解析时,可能需要对多组分的混合物进行详细分析,包括API详细的特征属性。
Reviewing the analytical toolkit for DPI de-formulation
干粉吸入剂配方解析的分析工具
FDA draft guidance highlights size, size distribution, morphological features and the crystal habit of an API as being among the Critical Quality Attributes (CQAs) for DPIs - those parameters that define clinical performance. An important aspect of demonstrating bioequivalence (BE)is having a detailed understanding of the way in which Critical Material Attributes (CMAs) of the formulation interact with device parameters to affect these CQAs.
FDA指导原则草案指出,API的颗粒大小、粒径分布、形态特征以及结晶习性均是干粉吸入剂的关键质量参数(CQAs),它们能决定干粉吸入剂的临床表现。而获取生物等效性(BE)的一个重要方法,就是要通过详细了解配方的关键物料参数(CMA)与设备参数两者之间的相互作用关系,从而进一步了解这些参数是如何影响关键质量参数(CQAs)的。
Cascade impaction is the technique specified in both the European and US Pharmacopoeias for measuring the aerodynamic particle size distribution of DPIs. This method size fractionatesthe dose delivered by a DPI on the basis of particle inertia, which is a function of aerodynamic particle size and particle velocity [3].The value of cascade impaction derives largely from its ability to deliver component specificAerodynamic Particle Size Distribution (APSD), which is obtained through the chemical characterisation of each individual size fraction. High Performance Liquid Chromatography (HPLC) is most usually employed for this purpose. This necessitates dissolution ofthe sample, meaning thatany information about the physical characteristics of particles within a specific size fraction is lost.
级联撞击技术可用于测量干粉吸入剂的空气动力学粒径分布状况,这在欧洲和美国药典中均有详细记载。该方法借助颗粒惯性对干粉吸入剂的传输剂量进行粒径分级,形成空气动力学粒径与颗粒运行速度[3]之间的函数。它的价值主要在于,当其对每一独立的粒径分级颗粒进行化学表征时,就能获得各种组分的空气动力学粒径分布(APSD)信息。而为了实现上述目标,研究人员通常采用高效液相色谱法(HPLC)对颗粒进行定量分析,但由于需要将样品进行溶解,研究人员将无法取得与特定组分颗粒相关的物理特征信息。
The technique highlighted by the FDA guidance for morphological studies within DPI development.is microscopy. This is an established technique butit too has limitations when it comes to efficientde-formulation.Conventional manual microscopy is labour intensive, timeconsuming anddoes not usually distinguish between the different components of a formulation.[2]
显微图像技术是FDA推荐用于研究干粉吸入剂开发过程中形态特征的一项技术。该技术已经发展成熟,但它在配方解析方面依旧存在局限性。例如,传统的人工显微技术耗时耗力,且一般无法对配方的不同组分进行区别鉴定。[2]
Complementary and alternative analytical techniques that can help to address these issues include laser diffraction, automated image analysis andMDRS, all of which can support and accelerate generic DPI development.
若想有效解决上述问题,研究人员可考虑采用替代或补充的分析方法,其中包括激光衍射技术、自动成像分析技术和拉曼结合自动成像技术等,这些技术都可支持并加快干粉吸入剂的仿制研发。
Laser diffraction – real-time monitoring of dispersion behavior
激光衍射 - 实时监控分散行为
Laser diffraction is an ensemble particle sizing technique that non-destructively delivers volume-based size distribution measurements across a particle size range of 0.01 – 3500µm. It is a highly automated and rapid method capable of measuring multiple particle size distributions in seconds. These attributes make laser diffraction highly complementary to cascade impaction.
激光衍射法是一种整体粒径测量技术,可对0.01– 3500微米粒径范围内的颗粒进行基于体积的粒径分布测量,且不会造成任何破坏。作为一种高度自动化的快速测量方法,它能够在数秒内测量多种粒度分布的情况。这些优点都足以让激光衍射法成为级联撞击技术的有效补充手段。
Laser diffraction measures the particle size of the whole formulation. It does notdeliver the component-specific information that cascade impaction provides, but it is far faster and provides valuable particle size data for the various stages of the actuation process.The capacity of laser diffraction for real-time analysis enables the measurement of particle size and concentration profiles for hundreds of device actuations in a single day.The technique can therefore be used to rapidly explore the dispersion behaviourof a DPI formulation and to correlate the effects of CMAs and device parameters with the efficiency of drug delivery [3]. Laser diffraction particle sizing provides detailed insight into dispersion dynamics, the efficiency of device emptying and the impact of flow rate on product performance (Figure 1). This information helps with scoping the design space for the product and supports therobust demonstration of BE.
激光衍射测量的是整个配方的粒径大小,无法像级联撞击技术那样给出特定成分的粒径信息。但它的测量速度要快得多,且能够提供吸入过程中不同阶段的粒径数据,而恰恰这些数据对于理解样品的输送过程是非常重要的。此外,激光衍射技术还能进行实时分析,因而可在一天内测量数百次触发过程中的粒径和浓度信息。因此,该技术可用于快速研究干粉吸入剂配方的分散行为,为关键物料参数和设备参数的结果与药物传输效率的关联研究提供支持[3]。采用激光衍射法进行粒度测量时,研究人员能够得到详细的分散动力学数据,并了解装置排空的效率以及流速对产品性能的影响(图1)。这些信息可帮助确定产品的设计空间,并为其生物等效性研究提供数据支持。
Boosting the informational productivity of cascade impaction
提高级联撞击技术的信息获取量
Automated image analysis enables statistically relevant morphological characterisation of a sample in a fraction of the time required to gather far fewer data by microscopy. With automated image analysis, tens of thousands of individual particle images can be captured in a matter of minutes. These are then used to determine morphological parameters for each individual particle to generate size and shape distributions for a sample. Such data support the detailed morphological investigation of a DPI dose, enabling a thorough study of large particles and agglomerates as advocated by the regulatory guidance. Increasingly automated image analysis is therefore replacing microscopy for this aspect of DPI characterization.
自动成像分析技术可对样本进行形态特征的统计分析。相较于普通的显微技术,自动成像技术不仅耗时更少,提供的数据也更丰富。研究人员可在几分钟内捕捉数万份单个颗粒图像,并利用这些图像确定每个颗粒的形态特征参数,以此得出样本的粒度与形状分布信息。这些信息使得研究人员能够对干粉吸入剂的形态特征进行仔细研究,从而对大颗粒和团聚物进行全面探究,而恰恰这样的全面探究正是监管指南中所提倡的。以上优势,让自动成像分析技术在干粉吸入剂表征领域逐步取代显微技术。
In addition, because automated image analysis is both relatively fast and non-destructive, it can provide important morphological information which is beyond the scope ofHPLC analysis of the size fractionated samples produced by cascade impaction. This strategy enables particle size and shape information to be gathered from various stages of a cascade impactor to develop a deeper understanding of how dispersion proceeds [4].
此外,当用级联撞击产生分级样本时,自动成像分析技术由于速度较快、无破坏性,它可提供HPLC无法提供的形态特征信息。这使得研究人员能够收集到级联撞击器在不同阶段的粒径和形状信息,进而对颗粒的分散过程形成更深刻的认识[4]。
Combining automatedimage analysiswithRaman spectroscopy enablesMorphologically Directed Raman Spectroscopy (MDRS), further extending this capability. With MDRS it becomes feasible to chemically identify individual particles and gather component specific morphological information for specific size fractions, within a DPI dose.
若是将自动成像分析技术与拉曼光谱相结合,一种全新的、能够增强颗粒分析能力的技术应运而生,即拉曼结合自动成像技术形态定向拉曼光谱(MDRS)技术。借助MDRS技术,研究人员可区分出单个颗粒的化学性质,并收集同一干粉吸入药剂中不同成分在不同粒径下的形态特征信息。
Case study: Using MDRS to gain insight into the dispersion behaviour of a DPI formulation containing two APIs
案例分析:使用MDRS技术深入了解含两种API的干粉吸入剂配方的分散行为
A sample of a commercially available dry powder inhaler containing two APIs was actuated into a Next Generation Impactor (Copley Scientific, UK) to disperse and fractionate the dose. A collection disk was placed in the collection cup of stage 3 of the impactor to capture particles on a suitable surface for MDRS. The collected dose was then transferred to a Morphologi G3-ID (Malvern Instruments Ltd, UK) for analysis.
研究人员将含有两种API的市售干粉吸入剂样本加入新一代撞击器(英国科普利科技有限公司产品)中进行分散和分级,并在撞击器中放置样品收集盘以捕获颗粒进行MDRS分析。随后,研究人员把收集到的颗粒转移至Morphologi G3-ID设备上(英国马尔文仪器有限公司产品)进行分析。
The deposited particles were initially characterised by automated image analysis to gather morphological information. From this analysis more than 1500 particles were then selected for chemical identification on the basis of their size and shape. Raman spectra were gathered for each of the selected particles and compared with a reference library containing spectra of the pure APIs and of lactose, an additional component in this formulation. This enabled secure chemical classification of the particles in the population of interest. A correlation score of 1 indicates that the particle has a spectrum closely matched to one of the pure components, while a lower score closer to zero, suggests an absence of that particular component.
沉积下来的颗粒最初通过自动成像分析技术进行表征以得到其形态特征信息。随后,研究人员从以上分析中选出1500多个颗粒,根据其粒径和形状进行化学鉴定。收集到所选颗粒的拉曼光谱后,研究人员将它们与仅含有API和仅含有乳糖(本配方中的添加成分)的参考光谱进行比对。通过以上操作,研究人员能够对目标颗粒进行可靠的化学分类。若相关系数为1,则表示该颗粒的光谱与纯API高度匹配;如果相关系数接近零,则表明这两种组分没有相关性。
Figure 2 shows an example spectrum for a specific particle, alongside reference spectra for the two active ingredients, and associated correlation scores. These scores suggest that this particle is amulti-component agglomerate (MCA), with clear spectral features associated with both APIs being observed within the particle’s spectrum. In a similar way, MCAs containing one or both APIs with lactose can also be identified.
图2为某特定颗粒的光谱与两种活性成分的参考光谱以及两者的相关性。相关系数表明,该颗粒是一种多组分的团聚物(MCA),其光谱特征与两种API关联性较高。通过类似的方式,研究人员可轻松识别出含一种或者两种API及乳糖的团聚物。
Figure 3 shows chemical identification data for the entire particle population of interest. These data show that of the particles captured on stage 3, and subsequentlyclassified as of interest, only around 1% are API 1 alone. The majorityare API2 but there are appreciable numbers of discrete lactose particles and multicomponent agglomerates. Example particle images are shown for each type of identified particle.
图3是所有目标颗粒的相关化学组分数据。这些数据表明第三阶段捕获的颗粒(即目标颗粒)中,仅约1%的颗粒只含有1号API。虽然大部分颗粒拥有2号API,但也含有相当数量的离散乳糖颗粒及多组分团聚物。图例中为每种已识别颗粒的图像。
These data provide far more insight into how the formulation is dispersed than does cascade impactionwith HPLC, which simply generates averaged chemical compositions for each size fraction. For example, they show whether both of the APIs detach from the lactose with equal ease and whether or not the APIs are likely to co-locate in the lung, on the basis of their size. This information is extremely helpful for understanding how a DPI delivers clinical efficacy and in demonstrating bioequivalence.
相比用HPLC进行级联撞击,以上数据能够更透彻地分析配方的分散过程,而前者仅能简单分析每一粒径级别的平均化学成分。通过粒径分析,这些数据可展示两种API是否同样容易从乳糖中分离,是否可能在肺部同一地点沉积。这些信息对于理解干粉吸入剂的临床效果、展示其生物等效性十分有用。
Working towards efficient de-formulation
实现高效的配方解析
De-formulation arguably presents an even greater challenge than formulation, most especially for complex products such as DPIs. Honing the analytical toolkit for DPI de-formulation is therefore essential. The traditional tools for inhaled product characterisation – cascade impaction and microscopy – have established benefits, but alternative techniques also have much to offer. Bringing speedand/or greater informational productivity, laser diffraction particle sizing, automated imaging and Morphologically Directed Raman Spectroscopy support the application of QbD and the efficient development of ANDAs.
配方解析比配方开发更具有挑战性,这对于干粉吸入剂等复杂配方产品来说尤为明显。因此,完善干粉吸入剂配方解析的分析工具就显得十分必要。用于吸入产品表征的传统工具——级联撞击器和显微技术——已被证实具有一定优势,但其他技术在这一领域也可大有作为。通过加快分析速度、帮助科研人员获取更多信息,激光衍射技术、自动成像技术以及拉曼结合自动成像技术能够支持质量源于设计(QbD)理念的应用,从而推动仿制药的有效研发。
References
参考文献
[1]http://online.wsj.com/news/articles/SB10001424052748703628204575618332675513808
[2]Guidance for industry. Metered Dose Inhaler and Dry Powder Inhaler Drug Products. Chemistry, Manufacturing and Control Documentation. Available for download at:http://www.fda.gov/downloads/Drugs/Guidances/ucm070573.pdf
[3] Kippax et al, “Unlocking the Secrets of the Dry Powder Inhaler Plume”, Proc. Drug Delivery to the Lungs 17, Edinburgh, 2006
[4] Huck D., Kippax P., Virden A., Kinnuen H., Shur J., Price R: “Improved understanding of the physical properties of DPI formulations by combining NGI size classification with Automated Image Analysis.” Drug Delivery to the Lungs 22, 2011, Conference Proceedings, 165-168.
Figures
图片
Figure 1: Powder concentration (Cv) versus Time profiles recorded for the emptying of a dry powder inhaler using laser diffraction. The data show that a higher concentration is delivered at high flow rates. This relates to the energy available for aerosolisation of this cohesive formulation.
图1:干粉吸入器在排空过程中,使用激光衍射技术捕捉的粉末浓度(Cv)与时间变化的关系。数据表明,当流速较高时,浓度也更高。而这与该粘性配方雾化时可提供的能量密切相关。
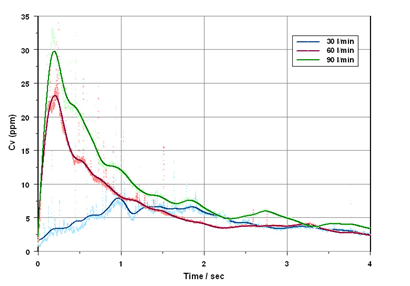
Figure 2: Comparing the Raman spectrum of individual particles with reference spectra for the APIs in the formulation enables secure chemical classification of the sample.
图2:将单独颗粒的拉曼光谱与配方中的API参照光谱进行比较,可对样本成分进行可靠的化学分类。
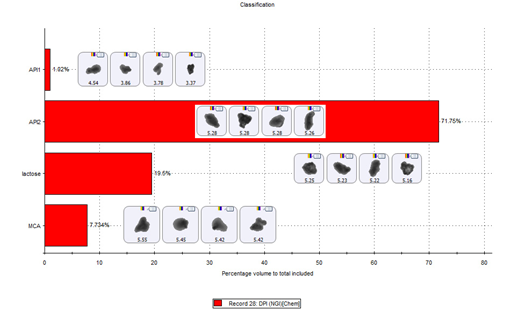
Figure 3: MDRS enables the precise classification of particles in an NGI sized fraction as being API, lactose or multicomponent agglomerates.
图3:在使用新一代撞击器对样本进行粒径分级时,采用MDRS分析法可将每一级别的颗粒准确分类为API、乳糖或含有多种成分的团聚物。
更多详情,请莅临:
中国国际粉体加工/散料输送展览会(IPB 2015)
展商:马尔文仪器
展位号:A 1508
时间:2015.10.28-30
地点:上海跨国采购会展中心 (上海市光复西路2739号)






